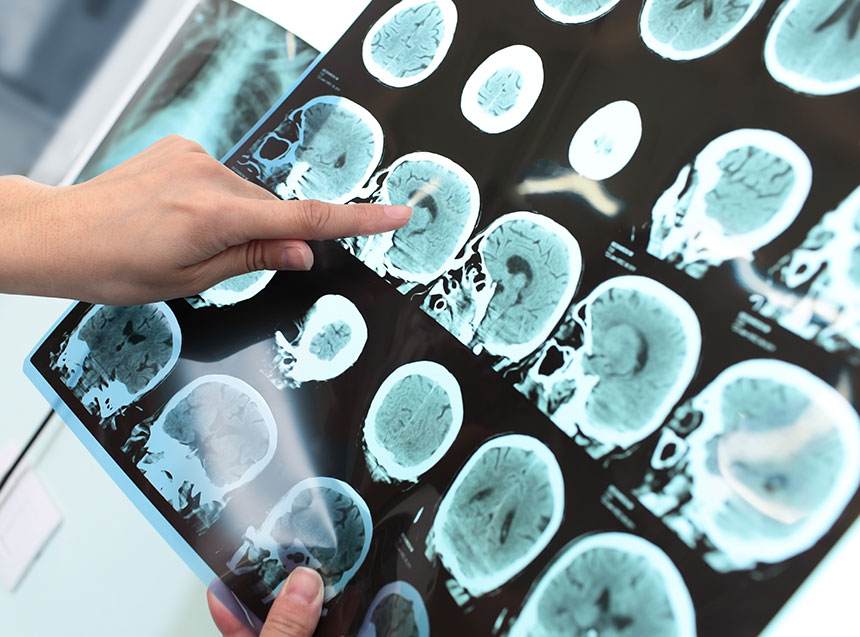
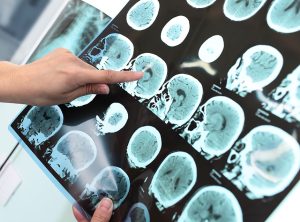
Amyloid plaques and tau (or neurofibrillary tangles) are the typical pathological lesions present in the shrinking brains of patients affected by Alzheimer’s disease. These pathological changes can be observed under a microscope, but the severity of the changes varies throughout the brain.
A recent study by Xu et al. (Scientific Reports, 9th June 2016) investigated the metabolic basis for these pathological changes that lead to Alzheimer’s disease. Xu et al. analyzed post-mortem brain tissue from nine Alzheimer’s patients and nine age-matched controls. They measured metabolite and trace metal levels in seven different brain regions from each patient and control. These seven brain regions are affected to varying degrees in Alzheimer’s patients, some severely affected (e.g. hippocampus), others mildly affected (e.g. sensory cortex) and one relatively spared region (cerebellum).
This study also measured fasting-plasma glucose and copper, and hemoglobin A1c in a case-control study of 85 participants. In the post-mortem study, the median weight of the affected brains was approximately 16% less than the median weight of the unaffected brains (from matched controls). This decline in brain mass is consistent with the histological severity and shrinking brains observed in numerous other Alzheimer’s disease studies.
Glucose is essential to generate energy in the brain and an impaired glucose uptake and utilization in the brain has been shown to be a key metabolic defect in Alzheimer’s patients. Interestingly, Xu et al. discovered that glucose levels were actually increased, rather than decreased, in all brain regions from Alzheimer’s patients. They proposed that the impaired glucose uptake is actually occurring in response to the elevated cellular glucose levels by down-regulation of the GLUT1 protein that is required to transport glucose across the cell membrane. Energy is normally obtained from glucose through glycolysis and the tricarboxylic cycles, but previous studies of Alzheimer’s patients have shown that defects occur in several tricarboxylic enzymes, thereby inhibiting this pathway. In contrast to the elevated brain-glucose levels, plasma-glucose levels in living Alzheimer’s disease patients were not elevated.
This confirms that these patients are not also suffering from a systemic disorder, such as type 2 diabetes. It also indicates that the defective glucose metabolism is restricted to the brain tissues and is not occurring elsewhere in the body. The polyol pathway is an alternative pathway that is activated if excess glucose is present, such as in diabetics. All the brain regions from Alzheimer’s patients also had elevated levels of two polyol pathway intermediates (sorbitol and fructose), showing that this alternative pathway metabolizes the excess glucose in the brains of Alzheimer’s patients. This may explain the link between elevated glucose and neurodegeneration, as glucose, fructose and sorbitol can each contribute to the formation of tissue-destructive advanced glycation endproducts (AGEs).
This study also proposes that the elevated glucose and polyol pathway may occur a considerable period before the onset of clinical symptoms. This was inadvertently discovered, when elevated glucose, sorbitol and fructose were detected in one of their unaffected control patients. Further analysis of this brain sample showed that although there had been no clinical evidence of dementia, the post-mortem analysis identified typical brain changes (plaques, tangles and reduced brain mass) confirming a preclinical Alzheimer’s diagnosis.
Previous studies of both sporadic and familial Alzheimer’s disease reinforce this hypothesis that elevated brain glucose is an early step in the disease progression. Copper is an essential trace metal for maintaining normal metabolic pathways and anti-oxidant defense. Each of the Alzheimer’s brain tissue samples had decreased copper levels compared to the unaffected controls, while the plasma-copper levels showed no difference between patients and controls. This again emphasizes that the Alzheimer’s-related changes are only occurring in a restricted area of the body – the brain.
Previous studies have shown that brain tissues of Alzheimer’s patients are modified by N-epsilon-carboxymethyllysine (CML) formation. CML is the link between the observed high glucose and low copper in affected brain tissue, as high glucose causes the CML-modification of collagen, which inhibits copper uptake. A similar high glucose-low copper ratio is observed in cardiac tissues of people affected by diabetes.
This study has described a new mechanism for the tissue destruction occurring in Alzheimer’s patients. This link between elevated brain glucose and defective cell-copper uptake is a potential reversible mechanism and is a possible new target for pharmacological developments.