One of the major changes observed in the brain of an Alzheimer’s patient is the accumulation of the beta-amyloid protein between the neurons. These toxic amyloid plaques prevent the essential synapses between the neurons and may also activate a damaging inflammatory response.
Several groups have been unsuccessfully attempting to use antibodies to selectively target and destroy these amyloid plaques. However, a recent study may have found the solution using an antibody called aducanumab. Aducanumab is a human monoclonal antibody that selectively reacts with beta-amyloid aggregates. Sevigny et al. recently published the very promising results of an aducanumab phase 1b clinical trial (Nature 537, 1st September 2016). In this trial, monthly doses of aducanumab resulted in a significant plaque reduction in early-stage Alzheimer’s patients.
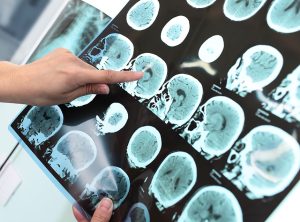
The 165 study participants were given either a placebo or varying doses of aducanumab through monthly intravenous infusions over a one-year period. Brain scans showed a plaque reduction over the treatment period, in patients that received aducanumab. The greatest reduction was observed in patients who received the highest dose of aducanumab. This plaque reduction was observed in patients who carried one or two of the increased risk (APOE e4) alleles, as well as patients who did not have the APOE e4 allele.
Some patients suffered adverse effects that included amyloid-related imaging abnormalities (ARIA), headaches, urinary tract and upper respiratory tract infections. In the patients that suffered from ARIA-E abnormalities (fluid build up in the brain), the problems typically resolved within 4 – 12 weeks, and no patients required hospitalization for this complication. This fluid build up was more common in patients with the APOE e4 allele and those that received a higher dose of aducanumab. The cause of ARIA-E is not fully understood, but may be due to underlying lesions in the blood vessel walls or an inflammatory response activated by the aducanumab antibody therapy.
This preliminary study was only conducted on a small sample from a limited region (USA only). Despite these limitations and potential side effects, this study showed great promise in the reduction of amyloid plaques over just a 12-month period, especially considering the plaques had likely been accumulating for over 20 years. Whether or not, this plaque reduction results in a cognitive benefit is currently unknown, but larger and more in-depth clinical trials of this drug are continuing and will run until at least 2020.
References: